15 batches of drugs are not in compliance!Involved in revision of pharmaceutical group Chanfukang Tablets, etc.
[ad_1]
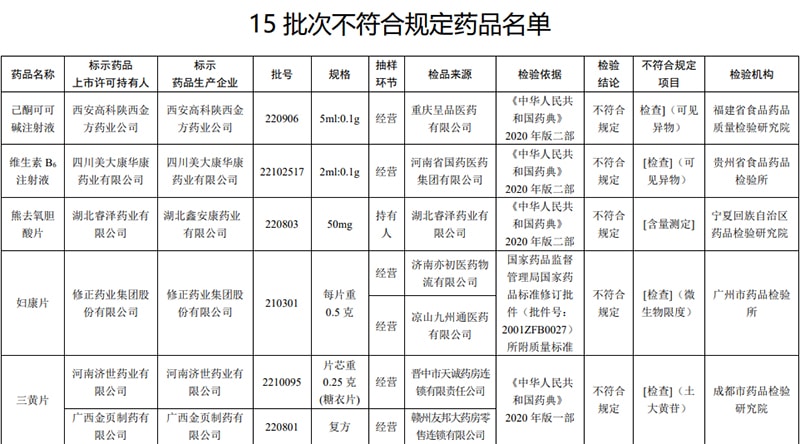
The State Food and Drug Administration recently issued a notice that 15 batches of drugs, including Fukang Tablets, produced by 15 companies including the Guangzhou Municipal Institute of Drug Control, were found to be non-compliant.
According to the notice, after inspection by the Fujian Food and Drug Quality Inspection Institute, a batch of pentoxifylline injection marked as Xi’an Gaoke Shaanxi Jinfang Pharmaceutical Company did not meet the regulations, and the items that did not meet the regulations were visible foreign objects.
After inspection by Guizhou Provincial Food and Drug Inspection Institute, a batch of vitamin B6 injection labeled as produced by Sichuan Meidakang Huakang Pharmaceutical Co., Ltd. did not meet the requirements, and the items that did not meet the requirements were visible foreign objects.
It is reported that visible foreign matter refers to insoluble substances that exist in injections and ophthalmic liquid preparations and can be observed under specified visual conditions, and their particle size or length is usually greater than 50 microns.
According to the inspection by Ningxia Hui Autonomous Region Institute of Drug Control, one batch of ursodeoxycholic acid tablets produced by Hubei Xin’ankang Pharmaceutical Co., Ltd. entrusted by Hubei Ruize Pharmaceutical Co., Ltd. did not meet the requirements, and the non-compliance items were content determination ( Content determination refers to the use of specified test methods to determine the content of active ingredients in raw materials and preparations).
After being tested by the Guangzhou Drug Control Institute, a batch of Fukang Tablets marked as Xiuxiu Pharmaceutical Group Co., Ltd. did not meet the requirements, and the items that did not meet the regulations were microbial limits.
It is reported that the microbial limit refers to the microbial control requirements for pharmaceutical preparations that do not directly enter the human body environment. Due to the slightly lower drug risk of such pharmaceutical preparations, a certain number of microorganisms can be allowed to exist, but some opportunistic pathogenic bacteria must not be detected. The microbial limit is divided into two parts: counting inspection and control bacteria inspection.
After the inspection by the Chengdu Institute of Drug Control, the two batches of Sanhuang Tablets marked as Henan Jishi Pharmaceutical Co., Ltd. and Guangxi Jinye Pharmaceutical Co., Ltd. did not meet the regulations. Whether there is rhubarb in the medicine of rhubarb).
After the inspection by the Chongqing Food and Drug Inspection and Testing Institute, it was marked as Sunflower Pharmaceutical Group (Xiangyang) Longzhong Co., Ltd. entrusted by Sunflower Pharmaceutical Group Hubei Wudang Co., Ltd. to produce a batch of Xingsu cough syrup that did not meet the requirements and did not meet the specified items. is the pH value (pH value is the hydrogen ion concentration index, as a measure of acidity and alkalinity).
Inspected by Zhejiang Food and Drug Inspection Institute, it is marked as Hebei Quantai Pharmaceutical Co., Ltd., Hebei Qixin Traditional Chinese Medicine Granules Co., Ltd., Shaoxing Zhenyuan Traditional Chinese Medicine Co., Ltd., Anhui Hongkun Pharmaceutical Co., Ltd., Shandong Bencaotang Traditional Chinese Medicine Co., Ltd. The 5 batches of fried jujube kernels produced by the company did not meet the requirements, and the non-compliant item was moisture (moisture refers to the water content in the drug. High moisture is usually related to factors such as craftsmanship, improper packaging, and storage and transportation environment).
According to the inspection by Ningxia Hui Autonomous Region Institute of Drug Control, two batches of Digupi produced by Chengdu Renjihong Pharmaceutical Co., Ltd. and Sichuan Guangran Chinese Herbal Pieces Co., Ltd. did not meet the requirements, and the non-compliance item was the total ash content.
According to the inspection by the Hebei Provincial Institute of Drug and Medical Device Inspection, a batch of poria cocos skin marked as produced by Anguo Ronghua Herbal Chinese Medicinal Materials Co., Ltd. did not meet the regulations. The non-compliant items included total ash and acid-insoluble ash.
It is reported that the purpose of total ash determination is to detect the purity of traditional Chinese medicine. The acid-insoluble ash index is mainly used to detect the content of impurities such as soil and sand in traditional Chinese medicine.
The State Food and Drug Administration stated that for the above-mentioned drugs that do not meet the regulations, the drug supervision and management department has required relevant enterprises and units to take risk control measures such as suspension of sales and use, recalls, etc., to investigate the reasons for the non-compliance and make effective rectification. The State Food and Drug Administration requires the relevant provincial drug supervision and management departments to organize investigations into suspected illegal activities of the above-mentioned enterprises and units in accordance with the “Drug Administration Law of the People’s Republic of China”, and disclose the results of the investigation and punishment in accordance with regulations.

Chart source: Screenshot of the website of the State Food and Drug Administration
[ad_2]
Source link

![[Love Wants Sexual Happiness Series 358]Find the culprit and overcome psychogenic erectile dysfunction. Don’t let pressure affect your sexual happiness.](https://chinathenews.com/wp-content/uploads/2024/04/171111-780x420.jpg)

![[Wanqingyi Care]My health, my rights, customized medical methods in the last stage of life](https://chinathenews.com/wp-content/uploads/2024/04/ZZ1-100-780x420.jpg)
![[Kidney Transplantation Special Topic]The survival rate of transplanted kidneys is high without dialysis treatment three times a week](https://chinathenews.com/wp-content/uploads/2024/04/1311-780x420.jpg)



